Tumor-specific colonization of engineered Sal and an evaluation of therapeutic efficacy
The Sal used on this work was the engineered pressure VNP20009, which the FDA has authorised for scientific analysis. It has steady proliferative exercise and favorable tumor-targeting properties. Transmission electron microscopy revealed the construction of the Sal as a blunt rod form with an approximate size of 1–5 μm (Fig. 1A). Cultivation in an LB liquid medium exhibited dynamic bacterial progress, monitored by UV absorbance at 600 nm. Inside 20 h, strong logarithmic progress was evident (Fig. 1B), indicating substantial proliferative exercise.
As a facultative anaerobe, Sal reveals a desire for colonizing tumor websites, however its non-specific distribution in regular tissue organs could trigger uncomfortable side effects. Utilizing a mouse 4T1 breast most cancers xenograft mannequin, we studied the in vivo distribution of the Sal after tail vein administration. We euthanized the mice and picked up the organs (coronary heart, liver, spleen, lung, kidney) and tumor tissues at numerous time factors post-administration. After processing, we assessed colonization by plating the micro organism on LB agar. Notably, 1 day post-administration, the considerable presence of Sal within the tumor tissue considerably surpassed that of the conventional organs (Fig. 1C, Determine S1), indicating preferential colonization and progress within the tumor microenvironment [27]. This will presumably be ascribed to the tumor microenvironment offering a extra favorable surroundings for micro organism (resembling hypoxia and a nutrient-rich, immunosuppressive microenvironment) [28,29,30,31]. Bacterial distribution was noticed in a number of regular organs after 4 days of injections, suggesting non-specific bacterial diffusion and distribution within the mice. Nonetheless, subsequent observations after 7 days revealed declining bacterial presence within the regular organs, which we attribute to innate immune clearance. The focus remained excessive within the tumor tissue, showcasing long-term colonization and tumor-specific results.
With a purpose to corroborate the tumor-targeting skills of Sal, we engineered it to emit pink fluorescence, and we marked the hypoxic marker CA9 with inexperienced fluorescence within the hypoxic tumor areas for co-localization evaluation [16]. Submit intravenous injection, the fluorescence staining of the frozen tumor tissues indicated substantial overlap between the Sal and the hypoxic tumor areas (Fig. 1D), confirming its enrichment in deep hypoxic tumor areas on account of its facultative anaerobic nature [16, 32]. These findings validate Sal’s means to selectively goal and colonize tumor areas whereas quickly clearing from regular organs; this ensures security for in vivo functions and establishes its viability for microbial immunotherapy in tumors.
With Sal’s concentrating on capabilities confirmed, we investigated its anti-tumor efficacy. The administration of various doses of Sal into the 4T1-bearing mice and monitoring the next tumor quantity enabled a dynamic analysis of therapeutic bacterial results. Notably, totally different doses of Sal displayed important tumor suppression in comparison with the management group (Fig. 1E). Nonetheless, an intriguing commentary emerged: the therapeutic impact didn’t exhibit a linear dose-dependent relationship. We enhanced tumor suppression effectivity by rising the bacterial injection dose from 105 to 106 per mouse. Nonetheless, past this level, additional dose increments led to diminished efficacy. The mouse survival curve evaluation echoed these findings (Fig. 1F). The dose-response sample of bacterial remedy differs notably from standard medication, and one potential motive is likely to be ascribed to the toxicity of the Sal at larger dosages. This necessitates additional exploration of the underlying mechanisms to domesticate a extra rational design for bacterial remedy doses and methodologies.
Characterization and antitumor actions of Sal. (A) TEM picture of Sal. (B) The kinetics of bacterial progress represented by OD600 values. (C) The colonization of Sal within the main organs of mice for various preparations examined by the unfold plate methodology (D) Immunofluorescence photographs of hypoxia and micro organism colocalization in tumor tissues at 48 h put up Sal injection. (E) The tumor progress curves of mice put up totally different remedies. (F) Survival charge of the mice with numerous remedies
Tumor-associated neutrophils as key regulators of Sal’s therapeutic efficacy
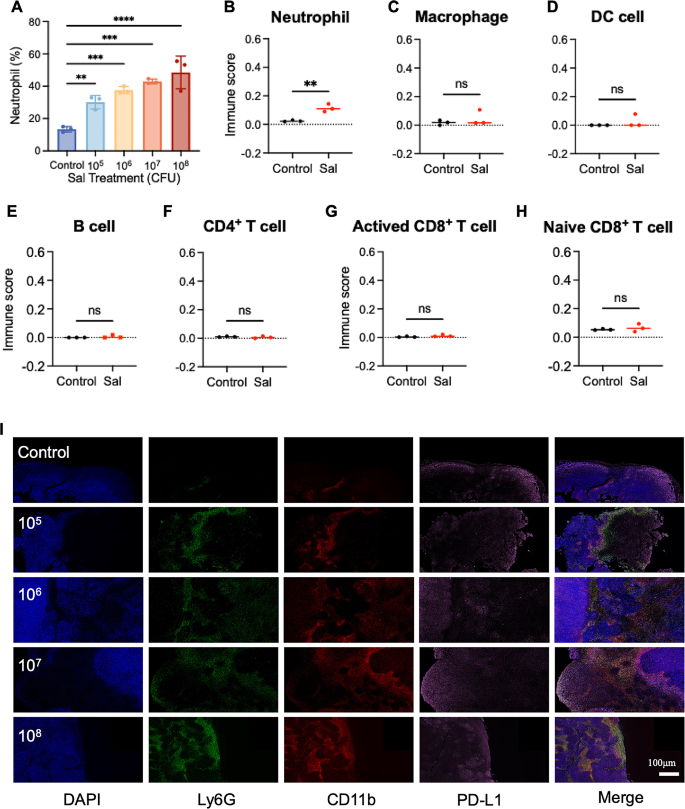
Whereas standard drug therapies typically exhibit dose-dependent developments inside outlined ranges, this conduct is just not evident within the case of Sal. We hypothesize that this deviation may hyperlink to the intricate anti-tumor mechanisms of Sal and its affect on the tumor microenvironment. As a overseas microorganism, Sal can incite the physique’s immune response towards an infection. Research point out that Sal colonization results in the substantial recruitment of neutrophils to the tumor web site [16]. But, the exact influence of neutrophil recruitment and activation on Sal’s therapeutic efficacy stays unclear. With a purpose to discover this, we carried out routine blood exams on tumor-bearing mice (post-treatment) underneath numerous Sal doses, revealing a proportional improve in neutrophil content material within the blood because the Sal dosage elevated (Fig. 2A), indicating micro organism’s direct activating affect on neutrophils.
Activated neutrophils possess the flexibility emigrate in direction of websites of bacterial colonization, prompting alterations within the immune microenvironment. With a purpose to probe this phenomenon, we carried out mRNA transcriptome sequencing on Sal-enriched tumor tissue, coupled with immune cell proportion evaluation utilizing the CIBERSORT deconvolution methodology. Submit-Sal therapy (Fig. 2B), we noticed a notable rise in neutrophil presence throughout the tumor microenvironment. Conversely, different immune cell varieties (Fig. 2C–H), resembling B cells, macrophages, DC cells, CD4+T cells, and CD8+T cells, confirmed no important numerical discrepancies, implying that neutrophils act as the first modulators of the Sal-colonized tumor immune microenvironment.
Earlier analysis signifies the various results of neutrophils on the tumor immune microenvironment, contingent upon their phenotypes [33]. Neutrophils can predominantly differentiate into both the anti-tumor N1 phenotype or the pro-tumor N2 phenotype. With a purpose to discover neutrophil phenotypic traits, we carried out immunofluorescence staining by using three markers—Ly6G (inexperienced fluorescence), CD11b (pink fluorescence), and PD-L1 (pink fluorescence)—within the handled tumor tissue Sect. [34]. Accompanied by the rise in Sal dose, we seen a considerable improve within the pink/inexperienced co-localization sign indicative of neutrophils (Fig. 2I), which is in step with the mRNA transcriptome sequencing outcomes talked about earlier. Furthermore, the co-localization of PD-L1 with neutrophils exhibited a notable improve, suggesting that neutrophils may predominantly undertake the N2 phenotype. This commentary means that though larger doses of Sal can recruit extra neutrophils, these cells primarily assume the N2 phenotype throughout the tumor microenvironment, doubtlessly compromising the anti-tumor impact. Consequently, modulating the neutrophil phenotype emerges as a pivotal technique for augmenting Sal’s anti-tumor efficacy by remodeling the tumor immune microenvironment.
The impact on tumor-associated neutrophils after Sal’s therapeutics. (A) Amount modifications of neutrophils within the blood after totally different remedies. (B-H) The estimated immune subpopulations after totally different remedies. Statistical check of immune cell scores by Scatter plot (unpaired t check). (I) The tumor immunostaining of Ly6G, CD11b and PD-L1 put up totally different remedies. Scale bar = 100 μm
Biocompatible MnO2 NPs as nano-activators of the STING pathway to induce IFN-β expression
The prior investigation has highlighted the pivotal position of tumor-associated neutrophil phenotypes in influencing the efficacy of Sal in anti-tumor remedy. Central to this affect is the promotion of the N1 phenotype of neutrophils whereas mitigating polarization in direction of the N2 phenotype. With a purpose to modulate neutrophil phenotypes successfully, we developed manganese dioxide nanoparticles (MnO2 NPs) for this examine. The design of the nanoparticles goals to activate the STING pathway throughout the tumor microenvironment by releasing Mn2+ intracellularly, thereby inducing IFN-β secretion and fostering polarization of neutrophils in direction of the N1 phenotype.
The MnO2 NPs had been synthesized by oxidizing Mn2+ underneath alkaline situations and by using hyaluronic acid (HA) as a template based on our earlier work [35]. The hydrated particle dimension of the MnO2 NPs measured roughly 200 nm (Fig. 3A), with an ζ potential of roughly − 17 mV (Fig. 3B). The UV absorption spectra illustrated a attribute shoulder peak throughout the 280–600 nm vary, validating profitable synthesis (Fig. 3C) [36]. AFM confirmed that the MnO2 NPs had been assembled from a number of small spherical particles with dispersed adhesion to the clustered HA template, making certain their stabilization (Fig. 3D and E). Elemental scanning and cross-sectional aspect statistics verified the distribution of the Mn, O, and N parts throughout the MnO2 NP construction (Fig. 3F,-H). The uniform dispersion of N parts throughout the particles additional corroborated the stabilizing impact of the HA. The morphological evaluation through SEM and TEM revealed that the ready MnO2 NPs had irregularly aggregated spherical buildings (Fig. 3I and J).
We carried out security assessments of the ready MnO2 NPs. The hemolysis charges at various concentrations had been under 5%, confirming the formulation’s security for intravenous injection (Determine S2). Cytotoxicity research revealed no important inhibitory results on the 4T1 tumor cells and DC cells inside a specified focus vary (Determine S3), indicating the excessive organic security of this nanoparticle formulation. The next in vivo evaluations assessed the potential of the MnO2 NPs to activate the STING pathway put up injection. The Western blotting evaluation demonstrated a major upregulation of phosphorylated STING protein (p-STING) and IRF3 protein (p-IRF3) within the tumor tissue of the mice following MnO2 NP therapy (Fig. 3Ok), confirming STING pathway activation by the MnO2 NPs. Moreover, ELISA assays revealed a considerable elevation within the IFN-β ranges within the serum of the mice underneath the affect of the MnO2 NPs (Fig. 3L), affirming the capability of the MnO2 NPs to stimulate IFN-β secretion by activating the STING pathway.
The characterization and STING pathway activating means of MnO2 NPs. (A) The scale distribution and (B) ζ potential of MnO2 NPs. (C) The UV-Vis spectra of HA, MnCl2 and MnO2 NPs. (D-E) The AFM microscopy of MnO2 NPs. (F-H) The basic scanning and cross-sectional aspect statistics of MnO2 NPs. (I) TEM picture and (J) SEM picture of MnO2 NPs. (Ok) The protein expression of p-STING, STING, p-IRF3 and IRF3 in tumor tissues of mice put up MnO2 NPs remedies detected by WB. (L) The extent of IFN-β within the serum of mice put up MnO2 NPs remedies detected by ELISA
In Vivo Regulatory Affect of MnO2NPs on Neutrophil Phenotype
Constructing upon the established position of MnO2 NPs in stimulating IFN-β secretion, we delved additional into their potential to modulate the phenotypes of neutrophils. With a purpose to assess this regulation, we carried out systematic staining of the floor markers of the tumor-associated neutrophils on the tumor tissue slices. The CD11b and Ly6G proteins served as markers for neutrophil localization, and CD54 (ICAM-1) and CD95 (Fas) had been indicators of the N1 phenotype [37,38,39,40]. Our findings point out a considerable improve in CD11b and Ly6G double-positive areas throughout the tumor area following Sal therapy (Fig. 4A), but we noticed no important alteration within the CD54/CD95 fluorescence alerts. Nonetheless, therapy with Sal plus MnO2 NPs remarkably elevated the alerts of CD11b, Ly6G, CD54, and CD95, suggesting the polarization of neutrophils in direction of the N1 phenotype upon their migration to the tumor tissue floor.
With a purpose to corroborate the influence of mixed Sal and MnO2 NP therapy on tumor-associated neutrophils, we carried out single-cell isolation from the tumor tissue and used move cytometry to establish their phenotype primarily based on floor markers. The outcomes post-Sal therapy indicated a notable discount within the proportion of neutrophils exhibiting the CD95/CD54 phenotype (Fig. 4B), indicating a predominant shift in direction of the N2 phenotype. Concurrently, a rise within the proportion of PD-L1-positive cells signified an immunosuppressive tumor microenvironment [34]. Nonetheless, upon mixed therapy with Sal and the MnO2 NPs, there was a major rise within the variety of CD54/CD95-positive cells, coupled with a lower within the variety of PD-L1-positive cells, confirming that Sal and MnO2 NPs have complementary results. We studied the dynamic change in neutrophil phenotype by co-staining CD54 and PD-L1. At 1 day post-Sal injection, neutrophils had been polarized into the N2 phenotype. Following the native administration of MnO2 NPs, extra neutrophils had been repolarized into the N1 phenotype, which was maintained for the next 4 days (Determine S4). Subsequently, Sal facilitated neutrophil aggregation within the tumor space, and the MnO2 NPs modulated the phenotype of the neutrophils.
Synergistic anti-tumor efficacy evaluation and mechanistic exploration of Sal and MnO2 NPs combinatorial remedy
Subsequently, we investigated the synergistic influence of Sal and the MnO2 NPs on tumor therapy on the animal stage. We dynamically monitored therapeutic efficacy all through the therapy by recording mouse tumor quantity (Fig. 5A). Notably, the MnO2 NPs exhibited modest anti-tumor exercise in comparison with the management group, which we attribute to the potential immune-regulatory impact mediated by manganese [41]. Conversely, Sal demonstrated substantial tumor inhibitory results; nevertheless, we noticed tumor regrowth after 14 days. Amongst all of the therapy teams, Sal plus MnO2 NPs displayed essentially the most pronounced anti-tumor impact, fully arresting tumor progress inside 20 days. The tumor tissues had been collected post-treatment for direct commentary, and the identical outcomes had been obtained (Fig. 5B). In line with the histological examination through H&E staining, we noticed a notable discount within the cell nuclei rely and elevated cell necrosis within the Sal plus MnO2 NPs therapy group (Determine S5). This signifies glorious anti-tumor efficacy. Survival curve evaluation additional validated the optimum therapeutic impact of Sal plus MnO2 NPs, attaining an 80% survival charge inside 40 days (Fig. 5C).
Our subsequent exploration aimed to elucidate the synergistic mechanism of Sal and MnO2 NPs. The mRNA transcriptome sequencing heatmaps depicted important alterations in gene expression upon including MnO2 NPs to the Sal therapy group (Determine S6). The neutrophil-related genes had been screened for enrichment, revealing a considerable improve in CCL3 gene expression for the Sal therapy teams (Fig. 5D). This means that the chemokines stimulated neutrophil enrichment within the tumor tissue. Moreover, measurements of the CCL3 ranges within the plasma and tumor tissue post-Sal therapy exhibited important upregulation (Fig. 5E and F), validating the aforementioned mRNA outcomes. It needs to be famous that CCL3 performs a pivotal position in tumor immunotherapy. CCL3 acts as a chemokine that not solely recruits neutrophils and promotes Th1 immune responses but in addition enhances the recruitment and activation of CD8 + T cells. These mechanisms collectively contribute to a strong anti-tumor immune response, underscoring the therapeutic potential of nanoparticle-induced CCL3 upregulation.
The sequencing outcomes additionally indicated a major elevation within the mRNA ranges of CD95 and CD54 following Sal mixed with MnO2 NP therapy when in comparison with Sal therapy alone. Equally, the experimental measurements displayed a marked improve in these markers pertinent to the anti-tumor neutrophils, resembling CD54, CD95, and CD16 (Fig. 5G-I), affirming the N1-oriented polarization impact of neutrophils underneath the affect of MnO2 NPs [34]. This may be defined by the numerous elevation of IFN-β gene worth which additionally validates the aforementioned STING pathway activating outcomes. Correspondingly, the sequencing and content material dedication confirmed a considerable rise in TNF ranges (Fig. 5J), signifying enhanced activation of anti-tumor immunity. The elevated variety of infiltrated CD8+ T cells detected through move cytometry throughout the mice tumors corroborates this impact (Fig. 5Ok). In abstract, the synergistic enhancement mechanism of Sal and MnO2 NPs is attributed to their regulatory influence on neutrophils, enhancing anti-tumor immune results and microbial immunity.
Lastly, we evaluated the biosafety of this therapy technique on the animal stage. There have been no important modifications within the weight of the mice throughout all therapy teams all through the therapy interval (Determine S7). The post-treatment serum evaluation of the biochemical indicators, together with ALT, AST, CRE, and BUN, confirmed that ordinary ranges had been maintained (Determine S8), indicating no liver or kidney toxicity. Additional histological evaluation of the most important organs within the mice through H&E staining (Determine S9) revealed no important pathological modifications post-treatment, highlighting good biosafety.
The synergistic anti-tumor efficacy and mechanistic Exploration of Sal and MnO2 NPs combinatorial remedy. (A) The tumor quantity of mice and (B) their pictures of tumor tissues and (C) the survival charge of mice put up totally different remedies. (D) Warmth map of genes associated to neutrophil phenotype based on the Database. (E) The extent of CCL3 in serum detected by ELISA and (F-Ok) the extent of CCL3, CD54, CD95, CD16, TNF-α in tumor tissues put up totally different remedies detected by q-PCR. (L) The variety of CD8+T cells in tumor put up totally different remedies detected by move cytometry