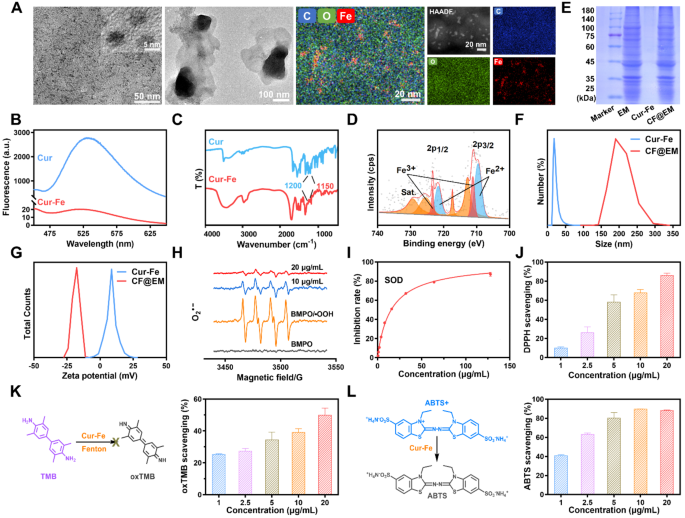
Synthesis and characterization of Cur-Fe and CF@EM
Cur-Fe was synthesized by dropwise addition of curcumin (Cur) to a mix of FeCl3 and polyvinylpyrrolidone (PVP). The obtained Cur-Fe considerably improved the solubility and dispersion of curcumin in water (Fig. S1A). The morphology of Cur-Fe was characterised utilizing transmission electron microscopy (TEM), revealing a uniform morphology and a dimension of roughly 5 nm. Power dispersive spectrometer (EDS) outcomes confirmed irregular doping of a major quantity of C, O, and Fe in Cur-Fe, suggesting that these parts are the primary elements of Cur-Fe (Fig. 2A). X-ray diffraction (XRD) evaluation additional confirmed that Cur-Fe is amorphous (Fig. S1B). Fluorescence emission spectra have been obtained for each curcumin and Cur-Fe at an excitation wavelength of 400 nm. The outcomes demonstrated that curcumin exhibited exceptional fluorescence at 520 nm, whereas Cur-Fe confirmed important quenching (Fig. 2B). This quenching impact could be attributed to the complexation between curcumin and the metallic ion, resulting in non-radiative cost switch. Fourier rework infrared (FTIR) spectra of Cur-Fe additionally indicated the coordination of ferric ions with the HO-C group in curcumin, as evidenced by the decreased infrared depth at 1150–1200 cm− 1 (HO-C stretching band) (Fig. 2C). UV-Vis outcomes revealed that Cur-Fe exhibited a most absorption wavelength just like that of Cur, in addition to a attribute absorption peak just like that of FeCl3 (Fig. S1C). These findings collectively counsel the formation of a steady advanced between Cur and iron ions within the nanomaterials. X-ray photoelectron spectroscopy (XPS) evaluation was carried out to find out the fundamental valence state of ferric ions within the nanomaterials, which confirmed the coexistence of Fe2+ and Fe3+ states (Fig. 2D). To acquire CF@EM, EM was added to Cur-Fe below ultrasound. TEM outcomes confirmed that a lot of black particles have been seen in CF@EM (Fig. 2A), indicating that Cur-Fe was efficiently encapsulated in EM. Equally, SDS-PAGE outcomes confirmed that the protein profile in CF@EM was according to that in EM (Fig. 2E). Dynamic gentle scattering (DLS) outcomes confirmed that the scale of Cur-Fe nanoparticles encapsulated with EM elevated from 19.12 ± 1.66 nm to 200.16 ± 17.35 nm (Fig. 2F), and the zeta potential decreased from 9.28 ± 0.88 mV to -13.81 ± 0.59 mV (Fig. 2G, S1D). All these outcomes indicated that CF@EM was efficiently synthesized. Concurrently, the scale of Cur-Fe and CF@EM have been measured over seven days, and no important adjustments have been noticed. This means that each Cur-Fe and CF@EM exhibit good stability (Fig. S1E).
Enzymatic actions of Cur-Fe
Since polyphenols are often thought-about pure antioxidants, to confirm the antioxidant capability of Cur-Fe, we evaluated its enzymatic exercise. O2•− is among the frequent free radicals, and hypoxanthine/xanthine oxidase (HYP/XOD) can produce O2•−, as a result of O2•− is extraordinarily unstable and exists for a really quick interval, BMPO was employed as a trapping agent to seize the produced O2•− and type a extra steady (BMPO/•OOH) spin adduct. Electron spin resonance (ESR) spectroscopy revealed that the O2•− produced by HYP/XOD could possibly be successfully scavenged by Cur-Fe (Fig. 2H). Nitro-blue tetrazolium (NBT) is a generally used O2•− tracer that reacts with O2•− to type water-insoluble formazan, and the O2•− scavenging skill could be measured by figuring out the absorbance of formazan at 560 nm. It was discovered that Cur-Fe had practically 90% O2•− removing at a focus of 20 µg/mL (Fig. S2). The SOD enzyme exercise of Cur-Fe was evaluated by testing with a commercially out there SOD assay package. It was discovered that Cur-Fe has a superb O2•− scavenging skill with an enzyme exercise of 269 U/mg (Fig. 2I). The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, one of many extensively used fashions in free radical scavenging assays, is a really steady nitrogen-centered radical. The outcomes confirmed {that a} decrease focus of Cur-Fe exhibited wonderful DPPH radical scavenging skill (Fig. 2J). 3,3’,5,5’-Tetramethylbenzidine (TMB) is a generally used •OH chromogenic substrate, and the •OH generated by the Fenton response can oxidize the TMB to oxTMB, and the Cur-Fe can scale back the oxTMB (Fig. 2Ok). Equally, 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulfonate) (ABTS) was generally used as a chromogenic substrate to detect the scavenging impact of ROS as a perform of the overall antioxidant capability of the nanoparticles. The outcomes confirmed that Cur-Fe with a decrease focus (5 µg/mL) might scavenge ~ 80% ROS, thus exhibiting wonderful antioxidant capability (Fig. 2L).
Characterization and ROS scavenging actions. (A) TEM pictures of Cur-Fe and CF@EM. The picture on the correct exhibits the ingredient mapping of Cur-Fe. (B) The fluorescence of curcumin and Cur-Fe. (C) FTIR of curcumin and Cur-Fe. (D) XPS of Cur-Fe. (E) SDS-PAGE protein evaluation of EM, Cur-Fe, and CF@EM. (F) Particle dimension distribution of Cur-Fe and CF@EM. (G) Zeta-potential of Cur-Fe and CF@EM. (H) ESR spectra of BMPO indicating O2•− scavenge with Cur-Fe. (I) SOD assay package to find out the SOD-like exercise of Cur-Fe. (J) DPPH radical scavenging ratio of Cur-Fe. (Ok) Schematic illustration of the TMB + •OH scavenging course of and the scavenging ratio of Cur-Fe. (L) Schematic illustration of the ABTS radical scavenging course of and the scavenging ratio of Cur-Fe. Information have been expressed because the imply ± SD (n = 3)
The intracellular anti-inflammatory and antioxidant results of Cur-Fe
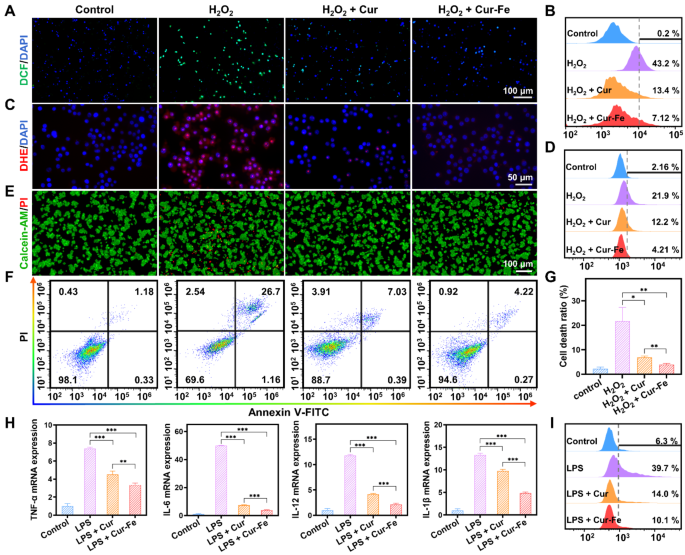
Underneath oxidative stress, reminiscent of stimulation with excessive concentrations of H2O2, cells generate giant quantities of ROS. 2,7-dichlorofluorescein diacetate (DCFH-DA) is a cell-permeable probe. It is going to be hydrolyzed to DCFH when it enters the cells, and non-fluorescent DCFH will likely be oxidized to fluorescent DCF by intracellular ROS. With H2O2 stimulation, robust inexperienced fluorescence appeared within the cells, indicating a considerable amount of ROS generated below the oxidative stress state. In distinction, the inexperienced fluorescence was attenuated by pretreatment with Cur and Cur-Fe (Fig. 3A). Circulation cytometry outcomes additional confirmed that Cur and Cur-Fe considerably decreased ROS ranges, and the scavenging skill of Cur-Fe was superior (Fig. 3B, S3A). To confirm the O2•− scavenging skill of Cur-Fe, dihydroethidium (DHE) was used to detect intracellular O2•− ranges after H2O2 therapy. Just like DCF, a considerable amount of pink fluorescence was produced within the cells after H2O2 stimulation, which was attenuated by pretreatment with Cur and Cur-Fe, and the scavenging capability of Cur-Fe was superior to that of Cur (Fig. 3C). The circulate cytometry outcomes have been according to the fluorescence pictures (Fig. 3D, S3B).
Extreme oxidative stress can induce oxidative harm and cell demise. To confirm the protecting skill of Cur-Fe towards H2O2-induced mobile oxidative harm, calcein-AM/PI was used to detect the mobile standing. Calcein-AM can simply penetrate the cell membrane of dwelling cells and emit robust inexperienced fluorescence, whereas PI can solely penetrate useless cells and emit pink fluorescence. After induction by H2O2, a proportion of cells died attributable to oxidative stress, and the variety of useless cells decreased after therapy with Cur and Cur-Fe (Fig. 3E). Detection of cell viability by methylthiazolyl tetrazolium (MTT) additional indicated that Cur and Cur-Fe considerably elevated cell viability after induction with totally different concentrations of H2O2, and the protecting impact of Cur-Fe on cells was extra pronounced (Fig. S4). Quantification of Annexin V-FITC/PI by circulate cytometry confirmed that Cur-Fe inhibited H2O2-induced apoptosis and thus enhanced cell viability (Fig. 3F, G).
Irritation is commonly accompanied by oxidative stress within the processes related to inflammatory illnesses. Lipopolysaccharides (LPS) can induce a wide range of cytokines and inflammatory mediators in vivo by activating macrophages, endothelial cells, and epithelial cells by means of cell signaling programs. Due to this fact, we used LPS to induce RAW 264.7 cells in vitro to simulate the acute inflammatory state. The relative expression of TNF-α, IL-6, IL-12, and IL-1β was decided by real-time quantitative polymerase chain response (qPCR). The expression of mobile inflammatory components was discovered to be elevated after LPS induction, whereas it was considerably decreased in each Cur and Cur-Fe teams in contrast with the LPS group, with anticipated, a extra pronounced lower within the Cur-Fe group (Fig. 3H). Equally, the flexibility of Cur-Fe to scavenge LPS-induced ROS was verified by incubating a DCFH-DA fluorescent probe to detect ROS technology in cells after LPS stimulation. The outcomes confirmed that the LPS group induced a better ROS technology, whereas each Cur and Cur-Fe teams decreased ROS technology (Fig. S5A). Circulation cytometry outcomes confirmed that Cur and Cur-Fe had related scavenging talents for LPS-induced ROS technology (Fig. S5B, C). The advanced tissue microenvironment can result in macrophage activation and polarization into functionally distinct subpopulations, and LPS induction often converts macrophages into M1 macrophages, which promotes irritation [32]. The impact of Cur-Fe on macrophage polarization was verified by observing adjustments in cell morphology and detecting CD86 overexpression on the floor of M1 macrophages. The outcomes of cell morphology confirmed that Cur and Cur-Fe inhibited macrophage morphology alteration (Fig. S6A). The outcomes of circulate cytometry equally confirmed that Cur and Cur-Fe inhibited the conversion of macrophages into pro-inflammatory M1 macrophages (Fig. 3I, S6B), thereby decreasing the expression of inflammatory mediators and assuaging mobile irritation. In the meantime, we in contrast the uptake of Cur-Fe and CF@EM on the mobile stage. The mobile uptake of Cur-Fe and CF@EM was additionally in contrast, and the outcomes demonstrated that the supply of Cur-Fe by EM enhanced the mobile uptake of Cur-Fe, as indicated by the elevated fluorescence depth of CF@EM noticed within the first hour. Moreover, the fluorescence intensities of Cur-Fe and CF@EM have been discovered to be related on the three-hour mark, suggesting that the mobile uptake of Cur-Fe had already saturated by then (Fig. S7). The presence of EM was noticed to don’t have any influence on the effectivity of Cur-Fe uptake by cells however moderately decreased the time required for saturation to be reached.
Anti-inflammatory and antioxidant results of Cur-Fe in vitro. (A, B) Fluorescence pictures (A) and circulate cytometry quantification (B) of DCF stained RAW 264.7 cells after totally different therapies. (C, D) Fluorescence pictures (C) and circulate cytometry quantification (D) of DHE. (E) Fluorescence pictures of calcein-AM/PI. (F) Circulation cytometry quantification of Annexin V-FITC/PI. (G) The cell demise ratio was calculated primarily based on the circulate cytometry knowledge in (F). (H) The mRNA expression of inflammatory cytokines (TNF-α, IL-6, IL-12, and IL-1β) in several teams. (I) Quantitative evaluation of M1 macrophages after totally different therapies by circulate cytometry. Information have been expressed because the imply ± SD (n = 3). Statistical evaluation was carried out utilizing a two-tailed Scholar’s t-test. *P < 0.05, **P < 0.01, and ***P < 0.001, represented totally different statistical significances, and ns represents no statistical distinction
Intestinal colonization of CF@EM
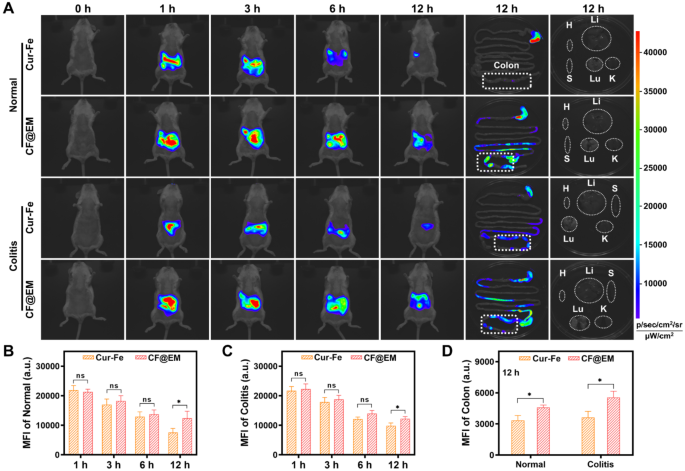
As a result of advanced atmosphere of the gastrointestinal tract, oral drug supply will encounter the degradation and absorption of medicine within the abdomen and small gut, leading to low drug concentrations within the colon, and shortened drug residence time as a result of peristaltic perform of the intestinal tract, which must be the potential causes of suboptimal drug remedy. To confirm intestinal colonization after Cur-Fe-coated EM, Cur-Fe and CF@EM have been administered to regular mice and DSS-induced colitis mice, respectively, after which their in vivo distribution was dynamically noticed. In regular mice, Cur-Fe was fully cleared from the physique inside 12 h, whereas CF@EM nonetheless had a robust fluorescence sign after 12 h, indicating that CF@EM was extra retained in regular mice (Fig. 4A, B). Comparable outcomes have been noticed within the UC mannequin. Though the altered gastrointestinal motility of the diseased mice resulted in much less Cur-Fe being incompletely cleared from the physique at 12 h, CF@EM nonetheless confirmed a robust in vivo retention capability (Fig. 4A, C). When the remoted tissues have been examined, it was discovered that CF@EM remained virtually fully within the gut, with the cecum and colon areas predominating, whereas CF@EM distribution was not detected in different tissues and organs (Fig. 4A). In contrast with the Cur-Fe group, the retention time considerably improved within the CF@EM group, and related outcomes have been present in each the traditional mannequin and the inflammatory situation, suggesting that CF@EM has a greater colonization impact on intestinal tissues (Fig. 4D).
Intestinal colonization of CF@EM. (A) Fluorescence pictures of regular mice, DSS-induced colitis mice, colon tissues, and organs at indicated time factors after oral Cur-Fe and CF@EM. H: coronary heart; Li: liver; S: spleen; Lu: lung; Ok: kidney. (B, C) Quantification of imply fluorescence depth in regular (B) and DSS-induced colitis mice (C) in vivo. (D) Quantification of imply fluorescence depth in colon tissues of regular and DSS-induced mice. Information have been expressed because the imply ± SD (n = 3). Statistical evaluation was carried out utilizing a two-tailed Scholar’s t-test. *P < 0.05, **P < 0.01, and ***P < 0.001, represented totally different statistical significances, and ns represents no statistical distinction
Biosafety evaluation of Cur-Fe and CF@EM
For the in vivo use of Cur-Fe and CF@EM, applicable biosafety evaluations must be carried out. The consequences of Cur-Fe and CF@EM on the viability of RAW 264.7 and FHC cells have been decided by MTT. The outcomes confirmed that cell viability was nonetheless round 80% after incubation of 200 µg/mL Cur-Fe or CF@EM with RAW 264.7 cells for twenty-four and 48 h. Equally, the identical focus of Cur-Fe or CF@EM was incubated with FHC cells for a similar time, and cell viability remained above 80% (Fig. S8A).
After oral administration of Cur-Fe and CF@EM, no important pathological adjustments in mice have been present in H&E staining main tissue sections (Fig. S8B). In the meantime, pink blood cells, white blood cells, lymphocytes, platelets, and hemoglobin have been examined, and the outcomes confirmed that there have been no important variations between mice within the Cur-Fe and CF@EM teams and mice within the management group (Fig. S8C). Equally, to judge the adjustments in biochemical indices, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) have been used as liver perform analysis indices, and creatinine (CRE) and urea have been used as renal perform indices, and it was discovered that each Cur-Fe group and CF@EM group confirmed no important variations in contrast with the management group (Fig. S8C). It was demonstrated that each Cur-Fe and CF@EM had good biosafety.
Therapeutic efficacy of CF@EM in DSS-induced colitis
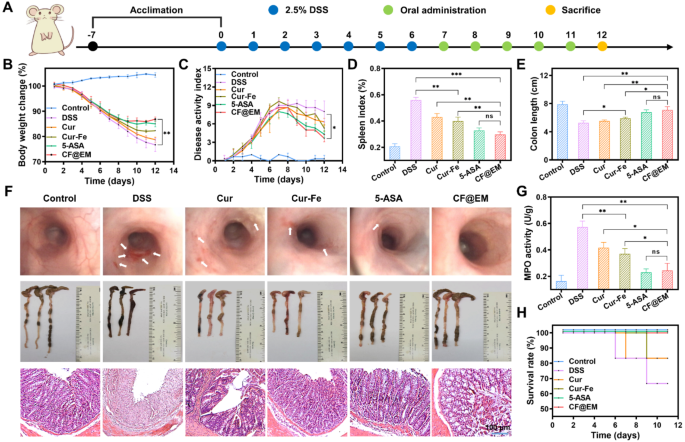
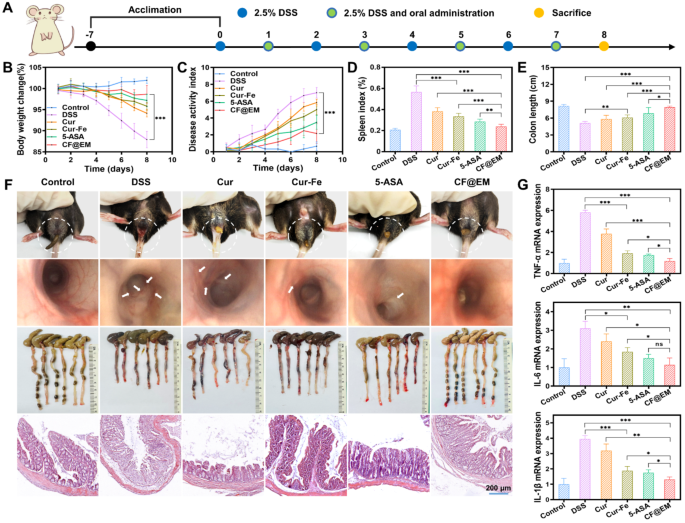
Based mostly on the intestinal colonization impact of CF@EM and the anti-inflammatory and antioxidant results of Cur-Fe, we evaluated the therapeutic impact of CF@EM in a DSS-induced ulcerative colitis mannequin. Based mostly on the outcomes of the biosafety evaluation, we determined to manage Cur-Fe and CF@EM at a dose of 30 mg·kg− 1. To raised consider the therapeutic impact of CF@EM, we chosen 5-aminosalicylic acid (5-ASA), a clinically used therapeutic drug, as a constructive drug management group. On the finish of seven days of modeling, drug therapy was administered for five consecutive days (Fig. 5A). The outcomes confirmed that the mice within the management group confirmed a pattern of gradual improve in physique weight and no important change in illness exercise index (DAI), whereas the DSS group confirmed a gradual lower in physique weight with the development of the illness, and the DAI was maintained at a excessive stage after repeatedly rising, indicating that the illness development was extra critical. In contrast with the DSS group, the remaining 4 teams confirmed a moderation within the diploma of weight reduction and improve in DAI, particularly the 5-ASA group and the CF@EM group confirmed a rebound in physique weight and a major lower in DAI after therapy, indicating that the illness was successfully managed (Fig. 5B, C). Endoscopic examination of the mice revealed that the intestinal mucosa of mice within the DSS group confirmed diffuse ulceration with hemorrhage, and the intestines have been considerably improved after therapy with CF@EM, and the impact was superior to that of Cur-Fe. The analysis of spleen index and colon size confirmed that the CF@EM group might considerably scale back the spleen index and restore the colon size, which was not considerably totally different from that of the 5-ASA group, whereas the straightforward Cur-Fe group had some therapeutic impact, however the efficacy was restricted (Fig. 5D-F). Within the H&E staining of the colon, it could possibly be seen that every one the epithelial cells and crypts have been destroyed within the DSS group, and likewise confirmed apparent inflammatory cell infiltration; the Cur and Cur-Fe teams preserved a part of the crypts and epithelial cell construction, and the lesion space was decreased. The CF@EM group considerably decreased the pathological harm of the colon (Fig. 5F). By myeloperoxidase (MPO) detection, it was discovered that each Cur-Fe and CF@EM might scale back the MPO stage, and the impact of CF@EM was extra important (Fig. 5G). Because the illness progresses, mice with extra extreme signs might die. Due to this fact, the survival charge of every group was counted. It was discovered that apart from the 5-ASA group and the CF@EM group, the survival charges of the opposite teams modeled by DSS have been decreased (Fig. 5H).
Therapeutic efficacy of CF@EM in DSS-induced colitis. (A) Schematic illustration of the institution and therapy of DSS-induced colitis mice. (B, C) Weight change curves (B) and DAI curves (C) of mice inside 12 days. (D) The spleen index of mice in several therapy teams. (E) The size of the colon. (F) Endoscopic pictures, colon pictures, and H&E staining of mice in several therapy teams. (G) The MPO exercise of mice. (H) The survival charge of mice in several therapy teams. Information have been expressed because the imply ± SD (n = 3). Statistical evaluation was carried out utilizing a two-tailed Scholar’s t-test. *P < 0.05, **P < 0.01, and ***P < 0.001, represented totally different statistical significances, and ns represents no statistical distinction
CF@EM relieves irritation and restores intestinal barrier perform
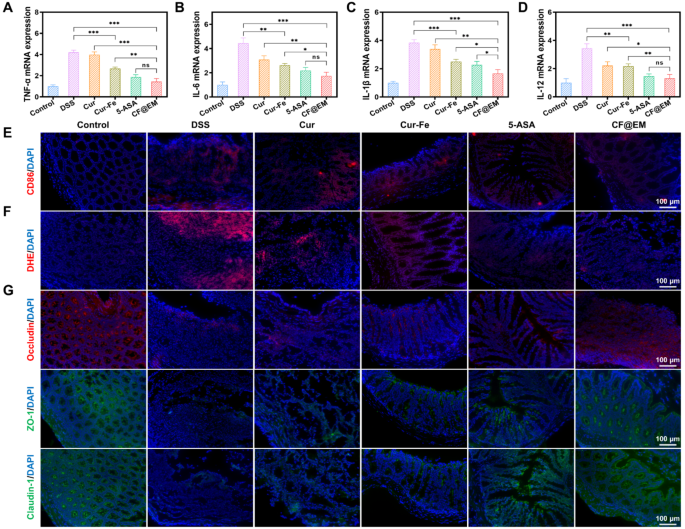
The manufacturing of inflammatory cytokines is essential within the growth of colitis. The detection of pro-inflammatory cytokines revealed that TNF-α, IL-6, IL-1β, and IL-12 have been extremely expressed within the DSS group, whereas Cur-Fe and CF@EM decreased the expression of pro-inflammatory cytokines, and the anti-inflammatory impact of the CF@EM group was considerably superior to that of Cur-Fe. The CF@EM group confirmed the identical anti-inflammatory capability in contrast with the 5-ASA group (Fig. 6A-D). Inflammatory cell infiltration is often one of many tissue options of colitis, and by immunofluorescence staining for CD86, a floor marker of M1 macrophages, we noticed a considerable amount of pink fluorescence within the colon of the DSS group. The expression of CD86 within the colon was dramatically downregulated after Cur-Fe and CF@EM interventions (Fig. 6E). These outcomes counsel that Cur-Fe and CF@EM can modulate macrophage polarization and scale back M1 macrophages that promote inflammatory responses, which helps to decelerate the event of colitis.
ROS usually accumulate in giant quantities at websites of irritation and trigger oxidative harm to cells. Fluorescence staining with DHE was carried out to judge O2•− technology in colon tissues. The outcomes confirmed that apparent pink fluorescence was noticed within the DSS group, indicating that DSS induced the technology of a considerable amount of O2•−, whereas the fluorescence depth was attenuated within the Cur-Fe group and the CF@EM group, and O2•− was successfully eliminated (Fig. 6F). These outcomes counsel that CF@EM can alleviate tissue harm in the course of the growth of colitis by decreasing intestinal ROS ranges.
The intestinal barrier is a crucial barrier that forestalls dangerous substances and pathogens from getting into the physique and maintains intestinal homeostasis [33, 34]. The paracellular permeability of the intestinal barrier will depend on tight junctions (TJ) between cells. By immunofluorescence staining of three tight junction proteins, occludin, ZO-1, and claudin-1, we noticed the adjustments in tight junctions after totally different therapies. It was discovered that the fluorescence of all three tight junction proteins was weak within the DSS group and elevated barely after Cur-Fe intervention. In distinction, the fluorescence depth was restored to virtually regular ranges within the CF@EM group (Fig. 6G), indicating that CF@EM can enhance intestinal barrier perform by restoring intercellular tight junctions.
CF@EM relieves irritation and restores intestinal barrier perform. (A-D) The mRNA expression of TNF-α (A), IL-6 (B), IL-1β (C), and IL-12 (D) in several teams. (E) Analysis of the M1 macrophage within the colon of the colitis mice after the therapy. (F) Fluorescence pictures of DHE. (G) Immunofluorescence picture of tight junction protein (occludin, ZO-1, and claudin-1). Information have been expressed because the imply ± SD (n = 3). Statistical evaluation was carried out utilizing a two-tailed Scholar’s t-test. *P < 0.05, **P < 0.01, and ***P < 0.001, represented totally different statistical significances, and ns represents no statistical distinction
Preventive impact of CF@EM in DSS-induced colitis
To additional validate the preventive impact of CF@EM, 4 teams of mice have been intermittently administered totally different preparations of the intervention over 7 days of oral DSS modeling (Fig. 7A). The outcomes have been as anticipated, with speedy weight reduction and better DAI scores within the DSS group, indicating sooner illness development and extra extreme signs. In distinction, the CF@EM group had virtually no important change in physique weight and decrease DAI scores, which have been considerably totally different from the DSS group (Fig. 7B, C). Remark of feces and endoscopy revealed that the DSS group had dilute liquid-like feces with apparent bleeding. The intestinal mucosa had a number of ulcers and was considerably shorter in size. Fecal standing and ulceration have been considerably improved after intervention with varied brokers, together with some restoration of spleen index and colon size. Particularly, the severity of colitis in mice was virtually maintained at a milder stage within the presence of CF@EM. There have been important variations in contrast with each Cur-Fe and 5-ASA teams (Fig. 7D-F). H&E staining revealed that the colonic crypts and epithelial cell construction have been much less broken within the CF@EM group, additional confirming that CF@EM had a helpful impact on inhibiting the event of colitis (Fig. 7F). After the detection of three pro-inflammatory cytokines, it was discovered that the expression of TNF-α, IL-6, and IL-1β was considerably downregulated in each Cur-Fe and CF@EM teams. It indicated that CF@EM had an inhibitory impact on the event of irritation and was considerably higher than Cur-Fe and 5-ASA (Fig. 7G).
Preventive impact of CF@EM in DSS-induced colitis. (A) Schematic illustration of the institution and therapy of DSS-induced colitis mice. (B, C) Weight change curves (B) and DAI curves (C) of mice inside 8 days. (D, E) The spleen index (D) and size of the colon (E) in several therapy teams. (F) Fecal Pictures, endoscopic pictures, colon pictures, and H&E staining of mice in several therapy teams. (G) The mRNA expression of TNF-α, IL-6, and IL-1β in several teams. Information have been expressed because the imply ± SD (n = 6). Statistical evaluation was carried out utilizing a two-tailed Scholar’s t-test. *P < 0.05, **P < 0.01, and ***P < 0.001, represented totally different statistical significances, and ns represents no statistical distinction
Modulating intestine microbiota by CF@EM
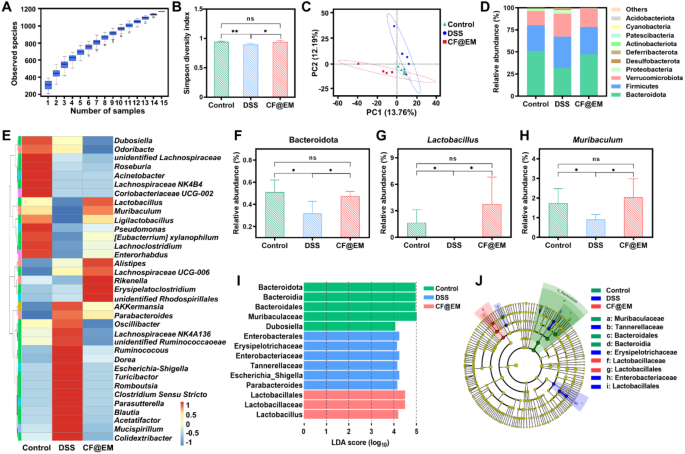
The intestine microbiota performs an vital position within the regulation of host metabolism, immunity, and intestinal barrier perform [35]. After observing the feces of mice, CF@EM was discovered to enhance the fecal standing induced by DSS. To confirm its regulatory impact on the intestine microbiota, we utilized 16S rRNA gene sequencing to investigate the adjustments. The species accumulation field plot demonstrated adequate pattern dimension and ample species richness (Fig. 8A). Species range serves as a priceless indicator of the structural and practical traits of a group, comprising α-diversity and β-diversity. We analyzed α-diversity by means of the Shannon index and the Simpson range index, and β-diversity by means of principal part evaluation (PCA). The Shannon index described the dysfunction and uncertainty within the incidence of people amongst species, whereas the Simpson range index mirrored the likelihood that two consecutive samples belong to totally different species. The outcomes confirmed that the Shannon index and the Simpson range index decreased within the DSS group, indicating a lower in α-diversity. Nonetheless, therapy with CF@EM elevated the decreased α-diversity attributable to DSS induction, according to the management group (Fig. 8B, S9). PCA displays the variations in multidimensional knowledge as distances in a two-dimensional coordinate plot by downscaling. The nearer the gap within the PCA plot, the extra related the group composition of the samples. The PCA outcomes confirmed that the group composition of the DSS group was totally different from that of the CF@EM group, suggesting that therapy with CF@EM altered the compositional construction of the intestine microbiota (Fig. 8C). Additional evaluation of species abundance on the phylum and genus stage revealed that the abundance of species within the DSS group was totally different from that within the CF@EM group (Fig. 8D, E). The abundance of Bacteroidetes is often intently associated to carbohydrate fermentation, nitrogen utilization, and bile acid biotransformation [36]. The abundance of Bacteroidetes was decreased after induction by DSS, and administration of CF@EM elevated its abundance within the intestine microbiota, with no important distinction within the CF@EM group in comparison with the management group (Fig. 8F).
Lactobacillus is a gaggle of gram-positive micro organism below the Lactobacillaceae of the Firmicutes, as a result of skill of Lactobacillus to enhance digestion and absorption, catabolize and metabolize bile acids, improve immunity, and inhibit irritation [37, 38]. It’s now extensively studied and used within the meals trade. The outcomes confirmed that the abundance of Lactobacillus was considerably decreased within the DSS group, and virtually all of them have been inhibited. In distinction, the abundance of Lactobacillus within the CF@EM group was restored to the management stage (Fig. 8G). The outcomes additionally confirmed that the abundance of Muribaculum was downregulated by DSS induction, whereas CF@EM considerably elevated its abundance and was not considerably totally different from the management group (Fig. 8H). Nonetheless, for Clostridium-sensu-stricto and Escherichia-Shigella, two teams of pathogenic micro organism, CF@EM confirmed a major inhibitory impact (Fig. S10) [39,40,41]. To additional clarify the noticed variations in microbial composition, linear discriminant evaluation impact dimension (LEfSe) was carried out. LEfSe was used to determine and interpret high-dimensional organic info, and after figuring out considerably totally different species by intergroup comparisons, the outcomes have been analyzed utilizing linear discriminant evaluation (LDA) to estimate the magnitude of the impact of every species’ abundance on the differential impact. Utilizing LDA (log10) > 4 because the cutoff worth, the outcomes confirmed that the pathogenic Escherichia-Shigella was considerably upregulated within the DSS group, whereas the helpful Lactobacillus was considerably elevated within the CF@EM group (Fig. 8I, J). Taken collectively, the therapeutic impact of CF@EM on colitis could be partially attributed to the modulation of the intestine microbiota, bettering bacterial range, and reworking the compositional construction of the microbiota to an anti-inflammatory phenotype.
Regulation of intestine microbiota by CF@EM. (A) The species accumulation boxplot. The horizontal coordinate is the variety of samples, the vertical coordinate is the variety of function sequences after sampling, and the general consequence displays the speed at which new function sequences seem below steady sampling. (B, C) The Simpson range index (B) and principal part evaluation (C) of the management group, DSS group, and CF@EM group. (D) The abundance on the phylum stage. (E) Heatmap of the relative abundance of the 35 most ample genus-level. (F-H) The abundance of Bacteroidetes (F), Lactobacillus (G), and Muribaculum (H). (I, J) linear discriminant evaluation impact dimension (LEfSe) and cladogram primarily based on LEfSe evaluation. LDA (log10) > 4 was used because the cutoff worth to point greater relative abundance within the corresponding group. In a cladogram, circles radiating from the within out symbolize taxonomic ranges from phylum to genus (or species). Every small circle at a distinct taxonomic stage represents a taxon at that stage, and the scale of the circle diameter is proportional to the relative abundance dimension. Species that aren’t considerably totally different are uniformly represented in yellow, and inexperienced, blue, and pink nodes point out microbial taxa which can be important within the management, DSS, and CF@EM teams. Information have been expressed because the imply ± SD (n = 5). Statistical evaluation was carried out utilizing a two-tailed Scholar’s t-test. *P < 0.05, **P < 0.01, and ns represents no statistical distinction
Regulation of intestine microbial metabolism by CF@EM
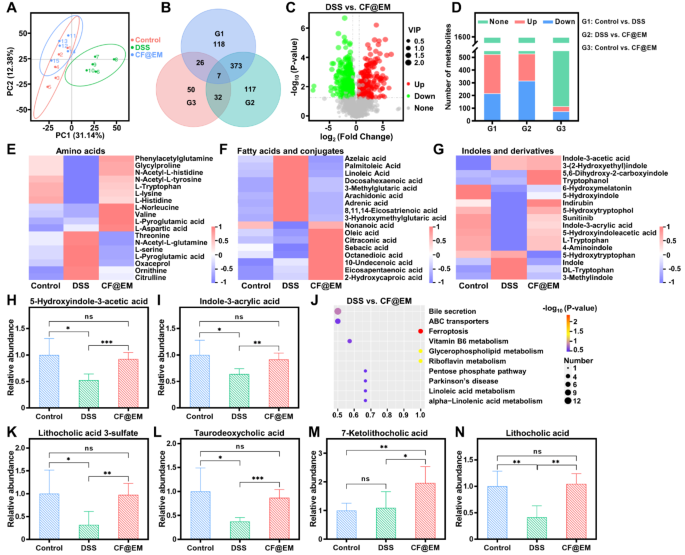
Intestine microbiota-host interactions are sometimes mediated by intestine microbial metabolites [42]. To additional examine the impact of CF@EM on intestine microbial metabolites after regulating intestine microbiota composition, we carried out fecal metabolomics evaluation. The PCA outcomes confirmed that the metabolite composition of the DSS group was considerably altered (Fig. 9A), indicating that the metabolites of the intestine microbiota have been altered by the induction of DSS. By evaluating the variations in metabolites between teams, which have been plotted as a Venn diagram and volcano plot, it was discovered that there have been a complete of 524 differential metabolites within the management group in contrast with the DSS group, and 216 metabolites have been considerably up-regulated and 308 have been considerably down-regulated within the DSS group. Whereas there have been a complete of 529 differential metabolites between the DSS group and the CF@EM group, 315 metabolites have been considerably up-regulated and 214 have been considerably down-regulated within the CF@EM group (Fig. 9B-D). It was discovered that the degrees of amino acids, fatty acids and conjugates, and indoles and derivatives confirmed totally different variations within the DSS group in comparison with the CF@EM group (Fig. 9E-G). Intestine microbiota can immediately convert fragrant amino acids into indole derivatives, which might preserve intestinal homeostasis by selling epithelial cell restoration, restoring intestinal barrier perform, and modulating immune cell perform [43,44,45,46]. 5-Hydroxyindole-3-acetic acid and indole-3-acrylic acid have been considerably decreased within the DSS group, whereas CF@EM restored them to manage ranges (Fig. 9H-I). The bile acid pathway was discovered to be considerably enriched by KEGG enrichment evaluation of differential metabolites within the DSS and CF@EM teams (Fig. 9J). Secondary bile acids together with lithocholic acid and taurodeoxycholic acid have been considerably decreased, which was improved by CF@EM (Fig. 9Ok-N). Bile acids and their derivatives have been proven to play an vital position in sustaining intestinal homeostasis. Bile acids attenuate intestinal irritation, modulate intestinal immunity, and enhance intestinal barrier perform [47, 48], and bile acid receptors mediate the regulation of intestinal irritation. Bile acid metabolism is considerably dysregulated in IBD, and fecal ranges of secondary bile acids are decreased in IBD sufferers [49, 50]. The above outcomes counsel that the therapeutic impact of CF@EM on colitis could also be inextricably linked to bile acid metabolism.
Regulation of intestine microbial metabolites by CF@EM. (A) The principal part evaluation of the management group, DSS group, and CF@EM group. (B) Venn diagram of differential metabolites for between-group comparability. (C) Volcano plot of DSS in contrast with CF@EM. (D) Metabolite adjustments have been in contrast between teams (whole metabolites = 1596). (E) Relative content material of amino acids between management, DSS, and CF@EM teams. (F) Relative content material of fatty acids and conjugates. (G-I) Relative content material of indoles and derivatives (G), 5-hydroxyindole-3-acetic acid (H), and indole-3-acrylic acid (I). (J) KEGG pathway enrichment evaluation of DSS in contrast with CF@EM. (Ok-N) Relative content material of lithocholic acid 3-sulfate (Ok), taurodeoxycholic acid (L), 7-ketolithocholic acid (M), and lithocholic acid (N) between management, DSS and CF@EM teams. Information have been expressed because the imply ± SD (n = 5). Statistical evaluation was carried out utilizing a two-tailed Scholar’s t-test. *P < 0.05, **P < 0.01, and ***P < 0.001, represented totally different statistical significances, and ns represents no statistical distinction