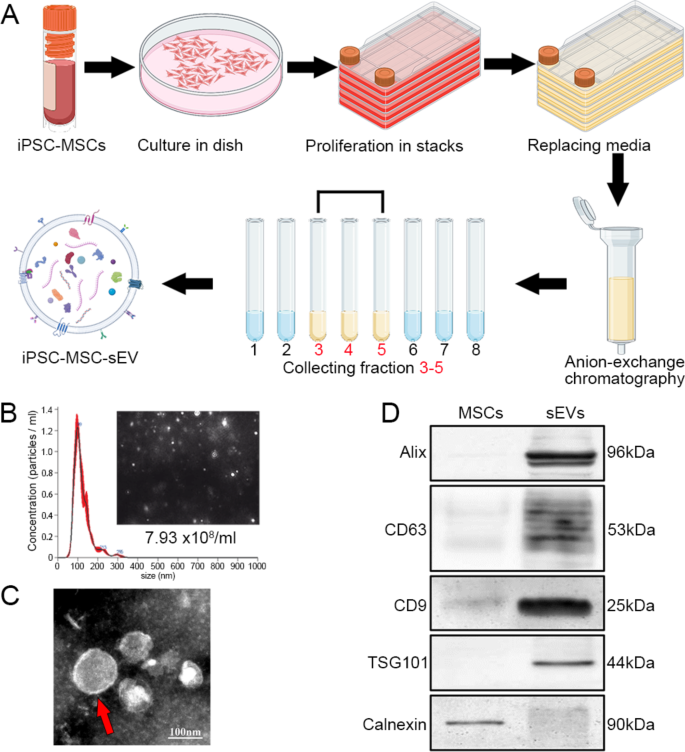
Preparation of high-purity sEVs from MSCs
We cultured iPSC-derived MSCs and purified sEVs from MSCs utilizing anion alternate chromatography (Fig. 1A). NanoSight detection of sEVs revealed uniform nanoparticles in sizes, with a peak at roughly 100 nm in diameter (Fig. 1B). Transmission digital microscopy confirmed that sEVs have been bilipid-layered nanoparticles (Fig. 1C). Western blots of sEVs and MSCs confirmed that sEVs have been constructive for Alix, CD63, CD9 and TSG101 and destructive for Calnexin (an endoplasmic reticulum–related protein not present in sEVs) (Fig. 1D). These outcomes point out the profitable technology of MSC-derived sEVs with excessive purity.
Purification and characterization of MSC-derived sEVs. A: Schematic of the preparation of iPSC-derived MSCs and the purification of sEVs from MSCs utilizing anion-exchange chromatography. B: NanoSight of sEVs confirmed a pointy peak of nanoparticle focus at about 99 nm in diameter; the typical diameter was 120.8 ± 2.2 nm and the focus was 7.93 × 108 nanoparticles / ml beneath the 200-times dilution. C: sEVs have been characterised as bilayer nanoparticles (crimson arrow). D: In western blots, sEVs expressed particular markers (Alix, CD63, CD9, and TSG101) and have been destructive for Calnexin; MSCs have been destructive for sEV-specific markers however constructive for Calnexin
Intranasally-delivered sEVs are broadly distributed within the mind and spinal wire of SOD1
G93A mice
Biodistribution of sEVs after intranasal administration was traced utilizing mCherry-labelled sEVs, which have been obtained from MSCs transfected with LV-CMV-mCherry-WPRE-pA throughout sEV preparation [24]. Fourteen-weeks previous SOD1G93A mice obtained mCherry-sEVs by way of intranasal supply day by day for two days, and mind and spinal wire tissues have been collected 24 h after the final administration. Within the sagittal sections, mCherry-positive particles have been seen within the olfactory bulb, cortex, crimson nuclei, and pons (Fig. 2A). Within the transverse spinal sections, mCherry-labelled sEVs have been primarily distributed within the grey matter and barely visualized within the white matter (Fig. 2B). In anti-ChAT immunostained sections, some spinal motoneurons have been nonetheless preserved within the ventral horn and stuffed with mCherry-labelled nanoparticles of their soma, as proven within the merged photographs (Fig. 2B). Spinal neurons have been visualized utilizing anti-NeuN immunostaining and lots of mCherry-labelled nanoparticles have been surrounding or in spinal interneurons (Fig. 2C). Ample reactive astrocytes have been distributed in the complete spinal wire of SOD1G93A mice and few mCherry-labelled nanoparticles have been ingested by astrocytes (Fig. 2D). Anti-Iba1 immunostaining confirmed a robust microglial response within the spinal part of SOD1G93A mice as indicated by microglia with enlarged soma and well-identified processes; mCherry-labelled nanoparticles have been present in some microglia (Fig. 2E). We additionally studied sEV biodistribution after intranasal administration of mCherry-labelled sEVs at day 120 and day 60 in SOD1G93A mice, which represented the later stage of ALS growth and the stage earlier than ALS onset respectively. At day 120, the biodistribution of mCherry-sEVs in vivo was much like that after intranasal administration at 14 weeks (Fig. S1). mCherry-sEVs have been additionally seen within the mind and spinal wire after intranasal administration at day 60 (Fig. S2), whereas the variety of mCherry-labelled nanoparticles was lower than that after intranasal administration on the later phases. These findings point out sEVs are effectively transported to the mind and spinal wire of SOD1G93A mice after intranasal administration and broadly penetrated into neurons and a few microglia, and that the penetration of sEVs into the CNS can also be affected by the ASL growth.
Biodistribution of sEVs within the mind and spinal wire ofSOD1G93Amice after intranasal administration. Fourteen-weeks previous SOD1G93A mice have been subjected to intranasal administration of mCherry-labelled sEVs day by day for two days and mCherry-labelled nanoparticles have been visualized within the mind and spinal wire sections. A: Within the sagittal part, mCherry-labelled nanoparticles (crimson) have been broadly distributed within the olfactory bub (OB, a1), cortex (Ctx, a2), diencephalon, and mind stem equivalent to crimson nuclei (RN, a3) and pontine (Pon, a4) nuclei. Neurons have been immunostained by anti-NeuN antibodies (inexperienced) and nuclei have been stained by DAPI (blue). B–E: Transverse lumbar spinal sections have been immunostained for ChAT (B), NeuN (C), GFAP (D), and Iba1 (E). Ample mCherry-labelled nanoparticles (crimson) have been seen within the grey matter, however not within the white matter. Within the anti-ChAT immunostained part (B), some spinal motoneurons (inexperienced) have been preserved within the ventral horn (VH) and stuffed with crimson nanoparticles of their soma (arrow) as proven within the photographs with excessive magnification (b1–b3). Anti-NeuN immunostaining labeled all spinal neurons (inexperienced, C), and crimson nanoparticles additionally encompass and/or penetrate spinal interneurons (arrow, c1–c3). GFAP-positive astrocytes (inexperienced) have been broadly distributed in the complete spinal wire (D), whereas few nanoparticles have been present in astrocytes (d1–d3). Within the anti-Iba1 immunostained part (E), some microglia (inexperienced) confirmed enlarged somas (arrows) and nanoparticles have been ingested by microglia (e1–e3, arrow). DH, dorsal horn. Identical scale bar in B–E
sEV administration prolongs survival time and improves motor operate in SOD1
G93Amice
SOD1G93A mice are the primary established ALS transgenic mouse mannequin; these mice grow to be paralyzed in a number of limbs due to motor neuron loss from the spinal wire and die by 5–6 months of age [25]. Irregular behaviors are detected in mice 12–14 weeks previous, indicating an early onset of pathological course of. To judge the therapeutic impact of sEVs on the illness development of ALS, sEVs have been intranasally delivered to 14-week-old SOD1G93A mice and continued as soon as each two days for one month; behaviors have been studied previous to and after the intervention (Fig. 3A). The management (PBS) group obtained the identical quantity of PBS. The physique weight of SOD1G93A mice was comparatively secure after administration in contrast with the baseline; there was no important distinction within the two teams regardless of a small lower within the sEV group at 18 weeks (Fig. 3B).
Progressive paralysis of skeletal muscle groups is a symptom of ALS. We measured grip power of limbs, calculated because the ratio to baseline, and noticed a fast lower from 14 weeks onwards within the PBS group (Fig. 3C). In distinction, the grip power within the sEV group confirmed a slight enhance at 15 weeks and was relative secure thereafter, with a major enhance in contrast with the PBS group at 18 weeks (Fig. 3C). In accelerating rotarod assessments, the falling latency of the PBS group progressively decreased from 15 weeks, whereas it didn’t considerably change in contrast with baseline within the sEV group (Fig. 3D). At 18 weeks, the falling latency (ratio to baseline) was 0.69 ± 0.06 within the PBS group and 0.92 ± 0.09 within the sEV group, with a major distinction between the 2 teams (Fig. 3D).
The mice within the PBS group exhibited paralysis at 18 weeks and thus we stopped behavioral assessments to keep away from any interference or undue stress. Within the PBS group, the primary mouse died at day 133 (19 weeks), and roughly 27% (3/11) of mice have been alive at day 168 (24 weeks). These survived mice have been very weak and failed to maneuver round freely for consuming, and we outlined 168 days (24 weeks ) because the endpoint of the experiment. In distinction, within the sEV group, the primary mouse died at day 164 and roughly 78% (7/9) of mice have been alive at day 168 (24 weeks). The survival price was considerably elevated within the sEV group in contrast with the PBS group (Fig. 3E). On the finish of the experiment, organs (liver, spleen, and kidney) have been collected; HE stain didn’t present any marked variations among the many three teams (Fig. S3).
Intranasal administration of sEVs slows motor operate decline and prolongs survival time inSOD1G93Amice. A: Experimental workflow of the timepoints of sEV or PBS administration and information acquisition. Behavioral research (animal weight, grip power, and falling latency on the rotarod) have been carried out previous to administration and as soon as every week thereafter. B: In the course of the 4 weeks of administration, mouse physique weight was comparable within the two teams. C: A lower in grip power was noticed from 14 to 16 weeks within the PBS group, whereas there was a slight enhance after which lower to baseline within the sEV group. D: Within the PBS group, the falling latency within the rotarod take a look at progressively decreased from 15 weeks. Within the sEV group, the falling latency barely elevated after one week of sEV administration after which was comparatively secure; latency was considerably larger in contrast with that of the PBS group. E: Survival curves. The share of surviving animals at day 168 (24 weeks) (dotted crimson line) was 27% (8/11) within the PBS group and 78% (7/9) within the sEV group, indicating a major enhance of survival chance within the sEV group. ***P < 0.001; ****P < 0.0001; ns, not important; two-way ANOVA a number of comparability in B–D; Gehan-Breslow-Wilcoxon take a look at in E
sEV administration reduces spinal motoneuron loss of life and synaptic denervation in SOD1
G93Amice
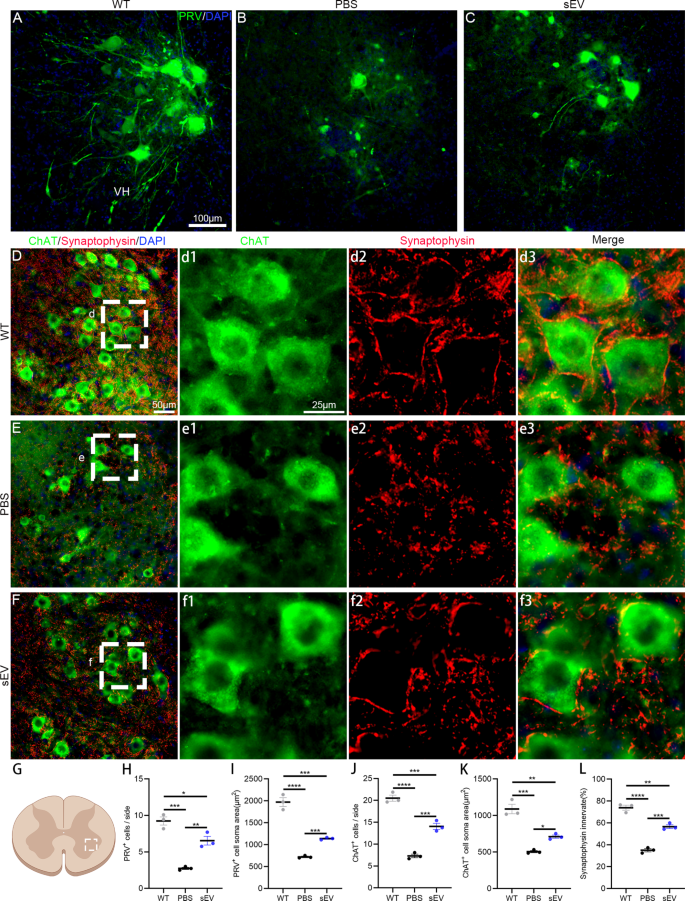
Motoneuron loss and axon denervation are typical pathological options within the ALS development. In mutant mice after 4 weeks of intervention and age-matched WT mice (18 weeks previous), PRV was unilaterally injected into sciatic nerves and retrograded-labelled lumbar spinal motoneurons have been analyzed on the ipsilateral aspect (Fig. 4A–C). Within the WT mice, many spinal motoneurons have been nicely traced and their dendrites have been readily visualized (Fig. 4A), whereas uncommon motoneurons within the PBS group and a few motoneurons within the sEV group have been noticed (Fig. 4B, C). The typical numbers of PRV-labelled motoneurons per part have been 9.2 ± 0.6 within the WT group, 2.7 ± 0.1 within the PBS group, and 6.5 ± 0.6 within the sEV group, with considerably decrease numbers within the PBS and sEV teams in contrast with the WT group and better numbers within the sEV group than the PBS group (Fig. 4H). The typical soma areas in PRV-labelled motoneurons have been totally different within the three teams; the soma space was largest within the WT group and smallest within the PBS, and the soma space of the sEV group was within the center (Fig. 4I).
To precisely evaluate surviving motoneurons and their synaptic innervation, lumbar spinal sections have been subjected to anti-ChAT and anti-synaptophysin double immunofluorescent staining and ChAT-positive motoneurons have been counted within the unilateral ventral horn of lumbar spinal segments (Fig. 4D–F). The typical ChAT-positive motoneurons per part have been 20.5 ± 0.7 within the WT group, 7.3 ± 0.4 within the PBS group, and 14.1 ± 0.7 within the sEV group, with important variations between the three teams (Fig. 4J). The typical soma areas of ChAT-positive motoneurons have been smaller within the PBS and sEV teams in contrast with the WT group, they usually have been bigger within the sEV group in contrast with the PBS group (Fig. 4Ok). Presynaptic inputs on spinal motoneurons have been recognized by anti-synaptophysin labelling (Fig. 4d1–d3, e1–e3, f1–f3); synaptic protection on particular person motoneurons was (73.9 ± 2.3) % within the WT group, (35.0 ± 1.4) % within the PBS group, and (56.6 ± 1.7) % within the sEV group, with important variations between teams (Fig. 4L). These outcomes point out that spinal motoneuron loss of life, axon degeneration, and synaptic denervation happen in spinal cords of SOD1G93A mice and sEV administration successfully diminished these pathological adjustments.
sEV administration delays motoneuron degeneration and synaptic denervation inSOD1G93Amice. A–C: PRV injection into sciatic nerves retrogradely labeled lumbar spinal motoneurons within the WT (A), PBS (B), and sEV teams (C). D–F: Anti-ChAT (inexperienced) and anti-synaptophysin (crimson) double immunofluorescent staining confirmed spinal motoneurons and presynaptic protection on spinal motoneurons within the WT (D, d1–d3), PBS (E, e1–e3) and sEV teams (F, f1–f3). Enlarged photographs in d1–d3, e1–e3, and f1–f3 are from the area indicated by the white dotted line in D, E, and F, respectively. G–L: Within the unilateral ventral horn of spinal wire (chosen area within the schema, G), statistical evaluation confirmed important variations in PRV-labelled motoneuron density (H) and common soma space (J), ChAT-positive motoneuron density (J) and common soma space (Ok), and the share of synaptophysin protection on motoneurons among the many three teams. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; one-way ANOVA a number of comparability; n = 3 mice in every group and 12–15 slides/mouse
sEV administration facilitates preservation of electrophysiological operate, myelin sheath, and NMJs inSOD1
G93Amice
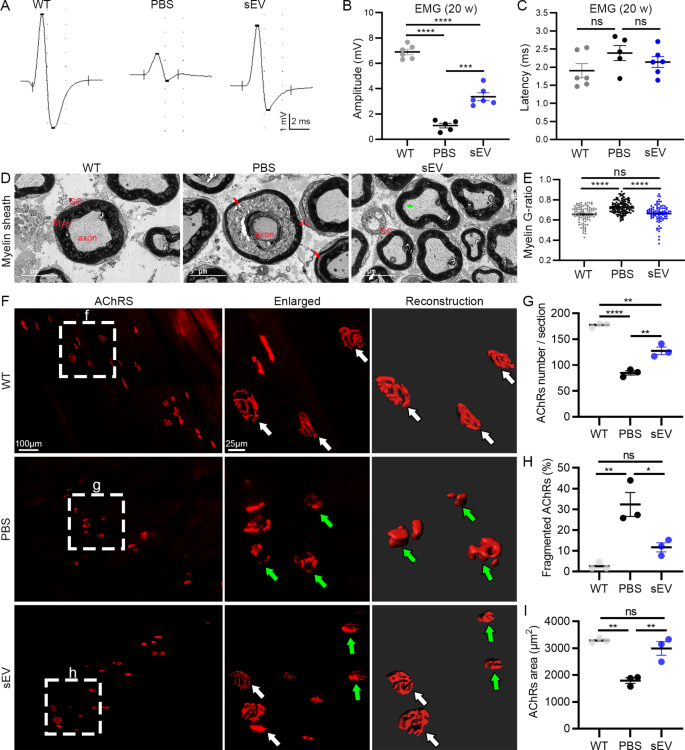
Spinal motoneuron axons innervate skeletal muscle groups and type NMJs to drive muscle motion. In SOD1G93A mutant mice, paralysis first appeared within the hindlimbs. Due to this fact, we recorded EMG of gastrocnemius upon stimulating sciatic nerves in 20-week-old animals (Fig. 5A). The amplitude of EMG was considerably decreased within the PBS ((1.08 ± 0.19) mV) and sEV ((3.36 ± 0.30) mV) teams in contrast with the WT group ((6.90 ± 0.22) mV), and it was considerably elevated within the sEV group in contrast with the PBS group (Fig. 5B). The EMG latency tended to be larger within the PBS group ((2.39 ± 0.21) ms) in contrast with the WT ((1.95 ± 0.20) ms) and sEV group ((2.14 ± 0.15) ms) however the distinction was not important (Fig. 5C). These outcomes point out sEV administration successfully reduces the decline in electrophysiological operate of spinal motoneurons in SOD1G93A mice.
After EMG recordings, mouse sciatic nerves have been collected for EM evaluation. In contrast with the WT group, through which the myelin sheath was nicely organized, axonal atrophy was readily recognized within the PBS group by folded and wrinkled myelin sheath and separation of myelin sheath lamellae stuffed with disintegrating particles (Fig. 5D). Within the sEV group, myelin sheath and axons have been preserved a lot better than within the PBS group; some separation of axon membrane and myelin sheath innermost lamellae was seen. The G-ratio of myelin sheath was considerably elevated within the PBS group in contrast with the WT and sEV teams, with no distinction between the WT and sEV teams (Fig. 5E). These outcomes point out that sEV administration inhibits axon demyelination and degeneration in SOD1G93A mice.
NMJ adjustments have been studied within the gastrocnemius by analyzing AchR clusters utilizing α-BT staining (Fig. 5F). Many fragmented AchR clusters have been recognized within the PBS group, whereas uncommon and a few clusters have been seen within the WT and sEV teams, respectively. The typical variety of AchR clusters was considerably decrease within the PBS group ((85 ± 4) clusters/part) in contrast with the WT group ((177 ± 3) clusters/part) and considerably larger within the sEV group ((127 ± 7) clusters/part) in contrast with the PBS group (Fig. 5G). The share of fragmented AchR clusters was larger within the PBS group ((32.3 ± 5.8) %) than within the WT ((2.5 ± 1.0) %) and sEV ((11.6 ± 2.2) %) teams (Fig. 5H). The typical areas of AchR cluster surfaces confirmed reverse adjustments within the three teams (in µm2: 3286 ± 48 within the WT group, 1789 ± 107 within the PBS group, 2988 ± 255 within the sEV group) (Fig. 5I).
sEV administration facilitates preservation of electrophysiological operate, NMJ AchRs, and myelin sheath inSOD1G93Amice. A–C: Consultant EMG recordings of gastrocnemius within the three teams (A); important variations have been noticed in amplitude among the many three teams (B), however no variations have been noticed within the EMG latency (C). D, E: EM research of sciatic nerves confirmed myelin sheath separation (crimson arrows) and axon atrophy within the PBS group and early myelin sheath separation within the sEV group (inexperienced arrow) however not the WT group (D). A major enhance of G-ratio was noticed within the PBS group in contrast with the WT and sEV teams, however no distinction was noticed between the WT and the sEV teams (E). F–I: AchR clusters within the gastrocnemius have been noticed by α-BT staining. Clusters have been nicely preserved (white arrows) within the WT group. Many clusters have been fragmented (inexperienced arrows) within the PBS group. Within the sEV group, some AchR clusters have been nicely preserved (white arrows) and a few have been fragmented (inexperienced arrows). Statistical evaluation confirmed important variations within the common AchR cluster numbers (G), the fragmented AchR cluster percentages (H), and the typical AchR cluster floor areas (I) within the PBS group in contrast with the WT and sEV teams. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not important; one-way ANOVA a number of comparability; n = 5 or 6 mice/group for EMG recordings and three mice/group for EM research and α-BT staining
sEV administration alleviates mitochondrial injury of motoneurons in SOD1
G93Amice
Mitochondrial injury is a crucial pathological attribute of human ALS sufferers and ALS animal fashions and is intently associated to excitotoxicity, apoptosis, and cell survival. To judge the impact of sEV administration on mitochondria, we carried out EM research of spinal ventral horns from 20-week-old mice. Within the WT animals, most mitochondria have been noticed within the cytoplasm of spinal motoneurons with rectangular or oval shapes, and mitochondrial cristae have been evenly distributed and connected by many high-density particles (Fig. 6A, A’). Within the PBS group, the morphology of mitochondria was fully deformed, displaying swelling and spherical shapes, and some cristae have been recognized in mitochondrial lumens (Fig. 6B, B’). Within the sEV group, some mitochondria additionally confirmed swelling, however the mitochondrial cristae and particles have been nonetheless preserved (Fig. 6C, C’).
To additional look at the adjustments of mitochondrial morphology, we measured the floor areas, circumferences, longitudinal and transverse diameters, and the areas stuffed with cristae within the mitochondrial lumens of all mitochondria in two well-identified motoneurons in every pattern (3 animals in every group). The typical floor areas of mitochondria have been (in µm2) 0.203 ± 0.013 within the WT group, 0.237 ± 0.005 within the PBS group, and 0.248 ± 0.006 within the sEV group, and there was a major enhance within the PBS and sEV teams in contrast with the WT group (Fig. 6D). The mitochondrial cristae areas have been additionally measured individually (in µm2): 0.0525 ± 0.0006 within the WT, 0.0174 ± 0.0002 within the PBS, and 0.0284 ± 0.0003 within the sEV, displaying the numerous variations amongst three teams (highest within the WT, center within the sEV, and lowest within the PBS; Fig. 6E). The mitochondrial circumferences have been elevated within the PBS and sEV teams in contrast with the WT group (in µm): 1.901 ± 0.034 within the WT group, 2.456 ± 0.030 within the PBS group, and a pair of.218 ± 0.034 within the sEV group; the circumference was smaller within the sEV group in contrast with the PBS group (Fig. 6F). The adjustments of those parameters point out the pathological traits of mitochondria in mutant mice. The side ratios of mitochondrial diameters have been 1.883 ± 0.036 within the WT group, 1.382 ± 0.010 within the PBS group, and 1.745 ± 0.025 within the sEV group, with a major enhance within the WT and sEV teams in contrast with the PBS group and no variations between the WT and sEV teams (Fig. 6G). Mitochondrial swelling was related to the lack of mitochondrial cristae and the vacuolation ratios (%) have been 8.470 ± 0.306 within the WT group, 42.191 ± 0.635 within the PBS group, and 18.16 ± 0.553 within the sEV group. The vacuolation ratio was considerably larger within the PBS group than within the WT and sEV teams, and the ratio was considerably decrease within the sEV group in contrast with the PBS group (Fig. 6H). These outcomes counsel that sEV administration successfully alleviates mitochondrial injury of spinal motoneurons within the pathological development of ALS in mice.
sEV administration alleviates mitochondrial injury of spinal motoneurons inSOD1G93Amice. A–C: EM photographs of spinal motoneurons from 20-week-old mice within the WT (A), PBS group (B), and the sEV teams (C). Mitochondria have been indicated by crimson arrows. A’–C’ are larger magnification photographs from the chosen areas in A–C, displaying particular person mitochondria. Nu, nucleus. D–H: Statistical evaluation of whole mitochondrial floor areas (D), mitochondrial cristae areas (E), circumferences F), side ratios (G), and vacuolation rations (H) within the WT, PBS, and sEV teams. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not important; one-way ANOVA a number of comparability; 80–150 mitochondria/neuron, 2 neurons/mouse, and three mice in every group for evaluation
sEV administration alleviates neuroinflammation in mutant spinal cords
Neuroinflammation is one other essential pathological function in ALS development. After 4 weeks of intervention in SOD1G93A mice, we collected spinal samples (lumbar enlargement segments) for cytokine detection. Among the many eight cytokines examined, the expression ranges of TNF-α, IFN-γ, and IL-10 have been comparatively constant in mice in the identical teams (n = 3/group) and have been larger within the PBS group in contrast with the WT and sEV teams (Fig. 7A). Quantitative evaluation revealed the expression ranges (in pg/mL) within the three teams (WT, PBS and sEV): TNF-α (91.55 ± 5.16, 113.90 ± 1.92, and 96.72 ± 5.65), IFN-γ (10.95 ± 0.68, 14.39 ± 0.44, and 12.02 ± 0.20), IL-2 (5.93 ± 2.46, 4.85 ± 0.13, and 5.23 ± 0.82), IL-4 (0.47 ± 0.17, 0.80 ± 0.15, and 0.31 ± 0.06), IL-5 (0.38 ± 0.05, 0.57 ± 0.09, and 0.37 ± 0.01), IL-10 (2.43 ± 0.44, 3.55 ± 0.14, and 1.83 ± 0.05), KC (0.97 ± 0.03, 1.35 ± 0.30, and 0.87 ± 0.13), and IL-12p70 (49.74 ± 16.75, 91.97 ± 13.26, and 42.54 ± 6.89). There have been considerably larger ranges of TNF-α and IFN-γ within the PBS group in contrast with the WT group (Fig. 7B), indicating a neuroinflammatory response in mutant animals. In distinction, the expression ranges of TNF-α, IFN-γ, IL-4, IL-10, and IL-12p70 have been considerably downregulated within the sEV group in contrast with the PBS group (Fig. 7B).
To additional affirm the neuroinflammatory adjustments in spinal cords, cervical spinal sections have been subjected to immunofluorescent staining towards Iba1 (for microglia) and GFAP (for astrocytes). Within the WT group, Iba1-positive cells have been evenly distributed within the white and gray matter (Fig. 7C), with small somas and lots of branches (Fig. 7c1–c3). Within the PBS group, elevated Iba1-positive cells amassed within the gray matter, significantly within the ventral horn (Fig. 7D), and these cells confirmed enlarged somas and shortened branches (Fig. 7d1–d3). Within the sEV group, fewer Iba1-positive cells and smaller somas have been recognized within the ventral horn in contrast with the PBS group (Fig. 7E). The microglia density within the ventral horn was considerably larger within the PBS group ((566 ± 22) cells/mm2) than within the WT group ((98 ± 3) cells/mm2) and the sEV group ((216 ± 18) cells/mm2) and barely larger within the sEV group than within the WT group (Fig. 7F). Equally, an elevated astrocyte response was recognized within the PBS group in contrast with the WT group, and this was decreased within the sEV group in contrast with the PBS group (Fig. 7G–I). The density of astrocytes within the ventral horn was considerably larger within the PBS group ((1116 ± 62) cells/mm2) than within the WT ((404 ± 16) cells/mm2) and sEV teams ((625 ± 28) cells/mm2) (Fig. 7J).
sEV administration alleviates neuroinflammation within the spinal wire of SOD1G93Amice. A, B: Cytokines have been analyzed in lumbar spinal samples after 4 weeks of intervention. Heatmap of the relative expression of cytokines in particular person samples (A). Quantitative analyses recognized a major upregulation of TNF-α and IFN-γ within the PBS group in contrast with the WT group and a major downregulation of TNF-α, IFN-γ, IL-4, IL-10, and IL-12p70 within the sEV group in contrast with the PBS group (B). C–F: Anti-Iba1 immunostaining confirmed microglial cells in transverse cervical spinal sections from the WT (C), PBS (D), and sEV teams (E). Photographs in chosen areas of three teams have been enlarged to determine microglial morphology within the corresponding left panels. Cell densities within the ventral horn have been larger within the PBS group than the WT and sEV teams and barely larger within the sEV group in contrast with the WT group (F). G–J: Astrocytes have been analyzed by anti-GFAP immunostaining within the WT (G), PBS (H), and sEV teams (I); astrocyte morphology within the ventral horn (chosen areas) was proven in enlarged photographs within the corresponding left panels. Cell density was larger within the PBS group than the WT and sEV teams and better within the sEV group in contrast with the WT group (J). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not important; one-way ANOVA a number of comparability; 3 mice in every group. DH, dorsal horn; VH, ventral horn
sEV administration inhibits overactivation of complement-coagulation cascade and NF-κB signaling pathways in SOD1
G93Amice
To decode the mechanism underlying results of sEVs in ALS mice, we collected lumbar spinal samples from the WT, PBS, and sEV teams (after 4 weeks of intervention) on the age of 18 weeks and carried out proteomics evaluation. A complete of 839 DEPs have been recognized within the PBS (mutant) group in contrast with the WT group, together with 506 upregulated DEPs and 333 downregulated DEPs (FDR < 0.05, foldchange > 1.5) (Fig. 8A). KEGG evaluation confirmed that 26 DEPs have been clustered within the complement and coagulation cascade (the highest pathway) and 14 DEPs have been clustered within the NF-κB signaling pathway (the highest nineteenth pathway) (Fig. 8C). The complement and coagulation cascades have been considerably overactivated in GSEA within the PBS group in contrast with the WT (normalized enrichment rating = 2.63, FDR < 0.00001) (Fig. 8E). All 26 DEPs within the complement and coagulation cascade, together with C1q, C3, C4b, Serpina1, Serpind1, Serping1, Plg, Itgb2, Itgam, A2m, F3, Clu, Cfd, Vtn, Cfi, Cfh, Fga, Fgb, and Fgg, have been considerably elevated within the PBS group (Fig. S4A). Overactivation of the NF-κB signaling pathway was additionally discovered within the PBS group in contrast with the WT (normalized enrichment rating = 1.92, FDR < 0.01) (Fig. 8G). Among the many 14 DEPs within the NF-κB signaling pathway, 12 DEPs (Nfkb2, Vcam1, Icam1, Irak4, Ddx58, Plcg2, Parp1, Lyn, Cd14, Trim25, Ighg2b, and Ighm) have been upregulated and a pair of DEPs (Traf3 and Tab3) have been downregulated (Fig. S4B). As well as, regulation of actin cytoskeleton was recognized as certainly one of prime 20 pathways in KEGG evaluation of DEPs between the PBS group and the WT group (Fig. 8C), and 30 DEPs have been clustered on this pathway together with 23 DEPs (Igtb2, Itga5, Actn1, Itga2, Egfr, Iqgap2, Vcl, Myh9, Nckap1l, Itgb5, Myl12a, Itga7, Itgb1, Fn1, Arpc1b, Iqgap1, Pak4, Itgam, Msn, Vav1, Fgf2, Ezr, Gm49368) with upregulation and seven DEPs (Pfn2, Limk1, Fgf1, Cfl2, Kras, Chrm2, Mylpf) with downregulation within the PBS group.
We additionally recognized 91 DEPs within the sEV group in contrast with the PBS group, together with 46 upregulated DEPs and 45 downregulated DEPS (FDR < 0.05, foldchange > 1.5) (Fig. 8B). KEGG evaluation confirmed that the complement and coagulation cascade (11 DEPs, prime 1st pathway) and the NF-κB signaling pathway (5 DEPs, prime twentieth pathway) have been considerably altered (Fig. 8D). These pathways have been considerably downregulated within the sEV group in contrast with the PBS group in GSEA (Fig. 8F, H). All 11 DEPs within the complement and coagulation cascade (C3, C1q, Serpina1a, Serpina1b, Serpina1e, Kng1, Plg, Fga, Fgb, Fgg, and Cfd) and 5 DEPs within the NF-κB signaling pathway have been decreased within the sEV group (Fig. S4C, D). Regulation of actin cytoskeleton was not recognized as among the many prime 20 pathways in KEEG evaluation of DEPs between the sEV and the PBS group.
Proteomic profiling of spinal wire samples in SOD1G93A mice after sEV administration. A, B: Volcano plots of differentially expressed proteins (DEPs) utilizing protein mass spectrometry evaluation of spinal wire samples within the mutant (Mut) group (particularly the PBS group) versus the WT group (A) and the sEV group versus the PBS group (B). C, D: KEGG evaluation recognized the highest 20 pathways of DEPs between the mutant (Mut) group and the WT group (C) and between the sEV group and the PBS group (D). The complement and coagulation cascade and NF-ĸB signaling pathways have been indicated by crimson arrows. E–H: GESA confirmed upregulation of the complement and coagulation cascade and NF-ĸB signaling pathway within the mutant group (Mut, particularly the PBS group) in contrast with the WT group (E, G) and downregulation of the complement and coagulation cascade and NF-ĸB signaling pathways within the sEV group (sEV) in contrast with the PBS group (F, H). Three animals in every group have been used for analyses
Western blots of spinal samples additionally confirmed a major upregulation of C1q and NF-κB within the PBS group in contrast with the WT group. Each C1q and NF-κB have been considerably downregulated within the sEV group in contrast with the PBS group (Fig. S5).
To look at whether or not adjustments of those signaling pathways additionally occur on the transcriptional degree, cervical spinal samples from the identical animals used for proteomics evaluation have been subjected to RNAseq. The outcomes confirmed 1438 DEGs together with 1112 upregulated DEGs and 326 downregulated DEGs within the PBS group in contrast with the WT group (Padj < 0.05, foldchange > 2) (Fig. 9A). The complement and coagulation cascade and NF-κB signaling pathways have been among the many prime 20 pathways within the KEGG evaluation of the full DEGs (Fig. 9B). Moreover, 18 DEGs (together with C1q, C3, C4b, Serpine1, F9, and Itgam) within the complement and coagulation cascade have been considerably upregulated within the PBS group in contrast with the WT group (Fig. 9C). Within the PBS group, 22 DEGs have been clustered within the NF-κB signaling pathway, together with 1 downregulated DEG (Card10) and 21 upregulated DEGs (together with Nfkb2 and Bcl2) (Fig. 9D). The outcomes additionally confirmed 26 DEGs together with 3 upregulated DEGs (Igsf9b, Lars2, and Gm23935) and 23 downregulated DEGs within the sEV group in contrast with the PBS group (Padj < 0.05, foldchange > 1.5) (Fig. 9E, F). Among the many 23 downregulated DEGs, 6 DEGs (C1qtnf7, C2, C1qtnf1, Serpina1e, Serpina3h, and Serpina9) have been concerned within the complement and coagulation cascade and a pair of DEGs (Bcl2l14 and Bcl2l1) have been associated to the NF-κB signaling pathway. The opposite 15 downregulated DEGs have been Ptgs2, Sult1a1, Xdh, Pnpla2, Plin4, Fkbp5, Fam214a, Dpep1, Zfand4, Hif3a, Card14, Sgk3, Tmem53, Fmo2, and Pla2g3 (Fig. 9F).
Transcriptomic profiling of spinal wire samples in SOD1G93A mice after sEV administration. A, E: Volcano plots of differentially expressed genes (DEGs) recognized by RNAseq of spinal wire samples within the mutant group (PBS group) in contrast with the WT group (A) and the sEV group in contrast with the mutant group (E). B: KEGG evaluation of the highest 20 pathways of DEGs between the mutant group and the WT group. The complement and coagulation cascade and NF-ĸB signaling pathways have been indicated by crimson arrows. C, D: Heatmaps of DEGs clustered within the complement and coagulation cascade (C) and the NF-ĸB signaling pathways (D) within the mutant group and the WT group. F: Heatmap of DEGs between the sEV group and the PBS group. Three animals in every group have been analyzed
Collectively, the proteomic and transcriptomic information demonstrated that the complement and coagulation cascade and NF-κB signaling pathways have been overactive within the spinal cords of SOD1G93A mice, and sEV administration alleviated activation of those signaling pathways.